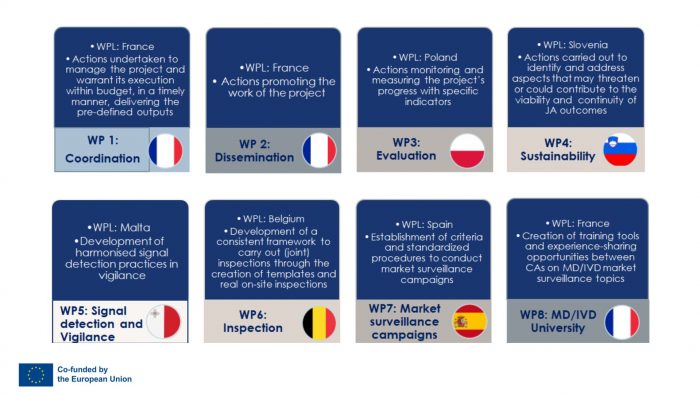
Understand more about Work Packages:
The Joint Action for Reinforced Market Surveillance of Medical Devices and In Vitro Medical Devices (JAMS 2.0) is divided into 8 Work Packages, all contributing to the achievement of the objectives set out in the project through defined tasks.
Work packages 1-4: Coordination, dissemination, evaluation and sustainability
These four WPs are “cross cutting”, involving activities to ensure the implementation and completion of the Joint Action.
The French Agency for the Safety of Medicines and Health Products (ANSM), main coordinator of the project, is responsible for the first two work packages:
- Coordination (WP1): ANSM is in charge of verifying that the Joint Action is progressing on time, within budget and with high–quality deliverables.
- Communication and Dissemination (WP2): ANSM has to establish actions promoting the work of the Joint Action and ensure visibility on its outputs. This WP aims to share information with all the relevant stakeholders.
The Office for Registration of Medicinal Products, Medical Devices and Biocidal Products (URPL) in Poland is in charge of the WP3:
- Evaluation (WP3): URPL has to monitor the actions of the project and measure its progress with specific indicators.
The Agency for Medicinal Products and Medical Devices of the Republic of Slovenia (JAZMP) is leading the fourth work package:
- Sustainability (WP4): JAZMP has to carry out actions to address aspects that may threaten or could contribute to the viability and continuity of JA outcomes.
Work package 5: Signal detection and Vigilance
As part of their work package to develop harmonised signal detection practices and vigilance, the Malta Medicines Authority (MMA) in Malta, in collaboration with other European beneficiaries, is in charge of:
- Determining and analysing the present state-of-the-art of signal detection in vigilance
- Establishing a team approach between Competent Authorities in developing and harmonising a framework for MD vigilance signal detection
- Developing the necessary guidance documents to support the integration and implementation of the framework within each Member State and at a European level
- Providing training to Member States on the implementation of the framework including capacity building and digitalisation
Work package 6: Inspection
As part of their work package to develop a consistent framework to carry out joint inspections, the Federal Agency for Medicines and Health Products (FAMHP) in Belgium is coordinating the following tasks:
- Harmonisation of the inspection activities throughout Europe
- Execution of joint inspections according to the developed framework
- Training of inspectors according to the framework.
Work package 7: Market Surveillance Campaigns
As part of their work package to establish criteria and set standardised procedures to conduct market surveillance campaigns, the Spanish Agency for Medicines and Health Products (AEMPS) in Spain is coordinating the following activities:
- Creation of tools to fulfil MDR/IVDR requirements that should be useful for the development of the European programs of market surveillance campaigns
- Development of methods for a joint, consistent and proactive approach to market surveillance campaigns as a means of underpinning the safety of the system
- Performance of a pilot campaign in order to validate the usefulness of the proposed criteria and methodology
Work package 8: MD/IVD University
As part of their work package to improve training tools and experience-sharing opportunities between CAs on MD/IVD market surveillance topics, the French Agency for the Safety of Medicines and Health Products (ANSM) in France, coordinator of the project, is responsible for the following tasks:
- Sharing of expertise between CAs on market surveillance (regulation, practices…) and of materials and tools already available at national and at European levels
- Development of training materials: Development of a training plan and training opportunities
- Creation of an EU academy platform to develop several training modules and use of available online platform (EU Academy/EU NTC/EU Health Policy Platform).
Co-funded by the European Union. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HaDEA). Neither the European Union nor the granting authority can be held responsible for them.